Elements in the alkali metals family will form ions with a charge of. D oxygen E nitrogen Answer.
2 Barium forms an ion with a charge of.
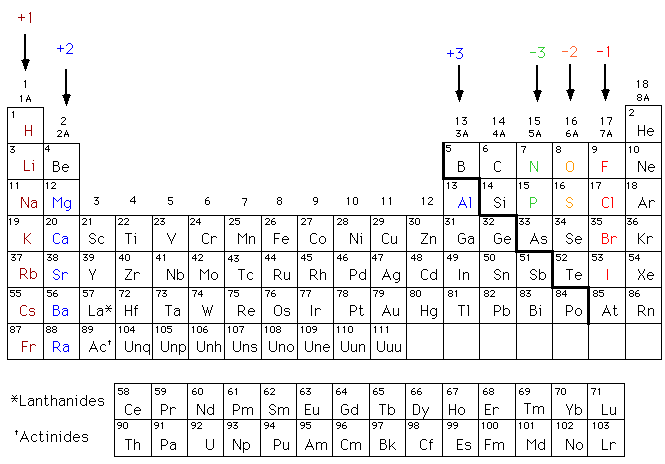
. These metals contain 2 valence electrons so in order to acquire a noble gas configuration they will lose their 2 electrons and attain a 2 charge. Sodium forms an ion with a charge of _____ 1 aluminum forms an ion with a charge of. A Alkaline earth metals B Halogens C Chalcogens D Alkali metals E Transition metals Answer.
All Group 2 Elements alkaline earth metals lose two electrons to form an ion with a 2 charge c. 3 energy levels and a Valence of 1. For example a neutral calcium atom with 20 protons and 20 electrons readily loses two electrons.
33 Votes To illustrate an atom of an alkali metal group 1 loses one electron and forms a cation with a 1 charge. Therefore B is the correct option. 2 bromine form an ion with a charge of.
Which of the ions or atoms below have the same electronic configuration as Ar. A molecule of water contains hydrogen and oxygen in a 18 ratio by mass. Therefore B is the correct option.
What is NOT one of the postulates of Daltons atomic theory. Which elements are likely to form ions with the same charge as chlorine. This is a statement of _____ atoms are composed of protons neutrons and electrons.
Here is the full list of metals in group one 1 charge. Law of constant composition. 3 calcium forms an ion with a charge of.
Chlorine typically forms an ion with a charge of -1. 455 2231 Views. 7 energy levels and a Valence of 3 b.
This problem has been solved. Lithium ion sodium ion potassium ion hydrogen ion silver ion ammonium ion copperI ion The element that forms a 2 plus ion with. B Alkaline earth metals.
Solution for typically form ions with a 2 charge A Halogens B Transition metals C Alkali metals D Alkaline earth metals Answered. 7 energy levels and a Valence of 5 d. A 11 _____ typically form ions with a 2 charge.
Group 6A nonmetals form -2 ions main group metals typically form ions with a noble gas electronic configuration Match each element with the predicted charge of its ion. B Alkaline earth metals. This column is commonly referred to as.
An alkaline earth metal group 2 loses two electrons and forms a cation with a 2 charge and so on. Which statement best explains why hydrogen forms ions that do not follow the octet rule. 22 _____ typically form ions with a 2 charge.
Typically forms ions with a 2 charge. The element in the Seventh family and Third period will have. All Group 17 Elements halogens gain one electron to form an ion with a 1- charge e.
Chlorine typically forms an ion with a charge of -1. _____ typically form ions with a 2 charge. Typically form ions with a 2 charge A bartleby close.
Here is the full list of metals in group two 2 charge. Group 2 elements are alkaline earth metals. All Group 16 nonmetals gain two electrons to form an ion with a 2- charge.
Group 13 metals like aluminum lose three electrons to form an ion with a 3 charge d. Which elements below are likely to form ions with the same charge as chlorine. These metals contain 2 valence electrons so in order to acquire a noble gas configuration they will lose their 2 electrons and attain a 2 charge.
3 energy levels and a Valence of 7 c. See the answer See the answer See the answer done loading _____ typically form ions with a 1- charge. Positively charged ions are called ____ while negatively charged ions are called _____.
_____ typically form ions with a 1- charge. Group 2 elements are alkaline earth metals. A Alkaline earth metals B Halogens C Chalcogens D Alkali metals E Transition metals.
All elements in column 2 of the periodic table consistently form ions with a charge of two plus. Alkaline earth metals B.

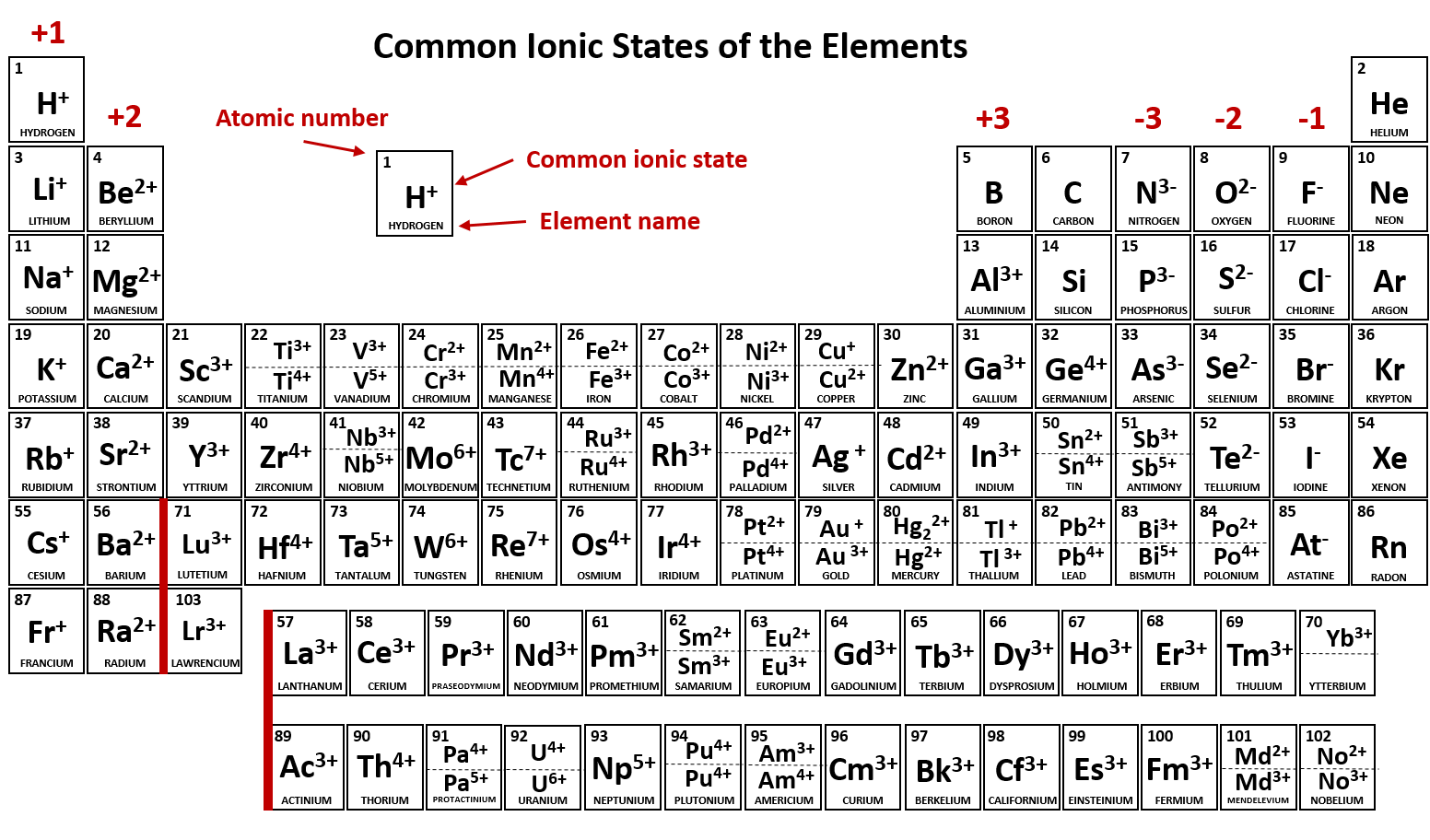
0 Comments